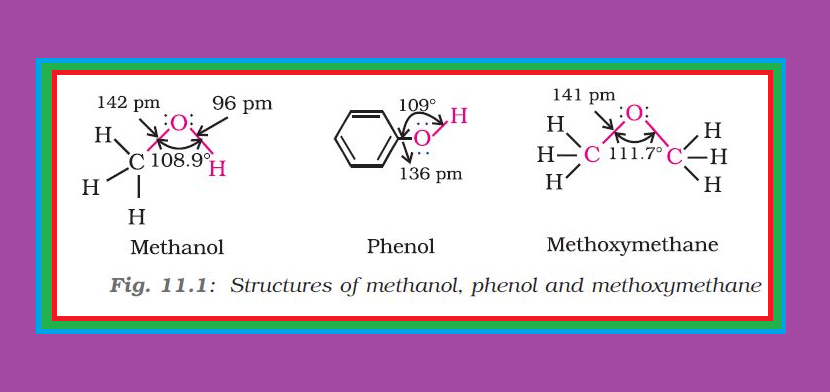
Alcohols :
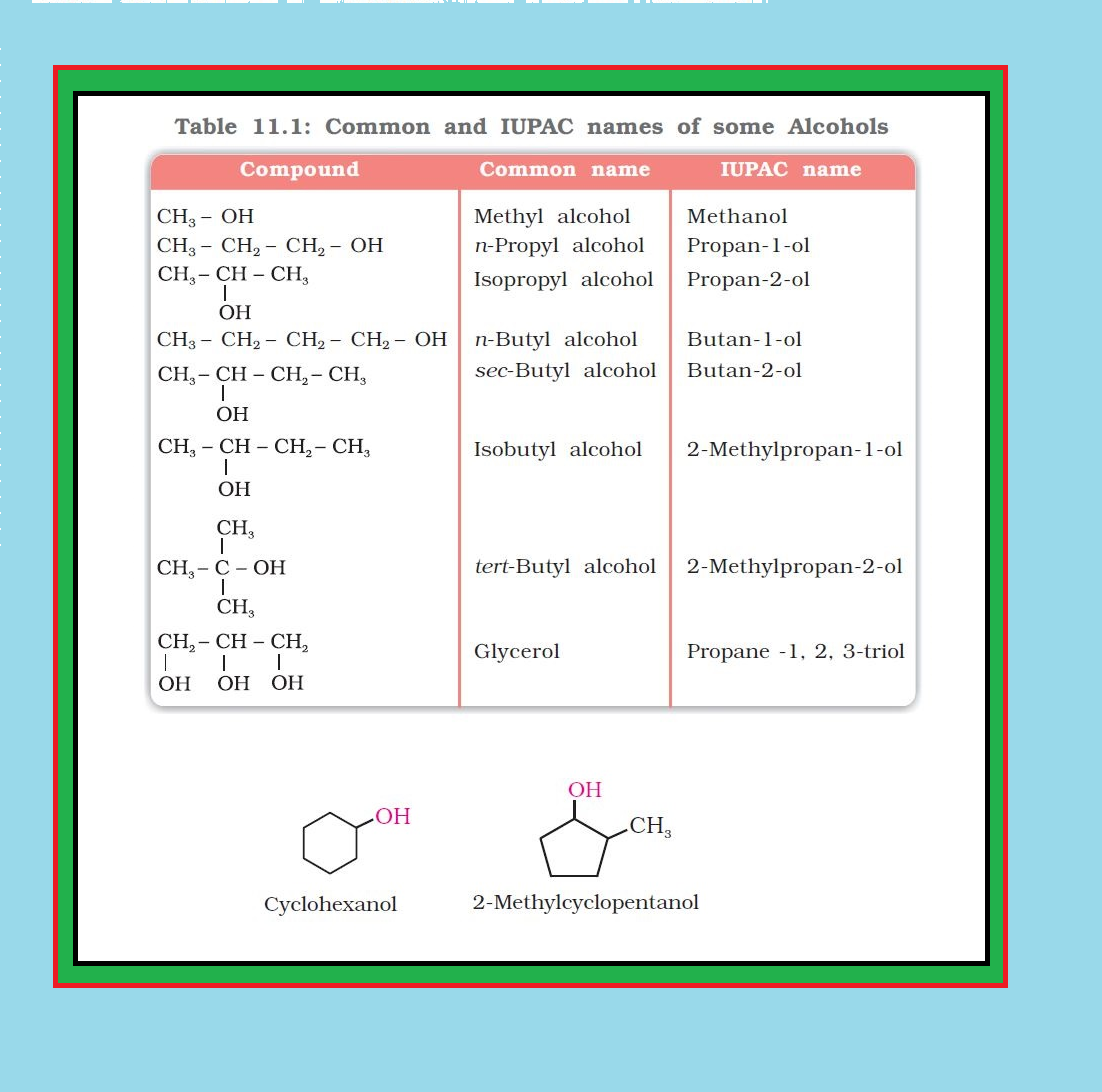
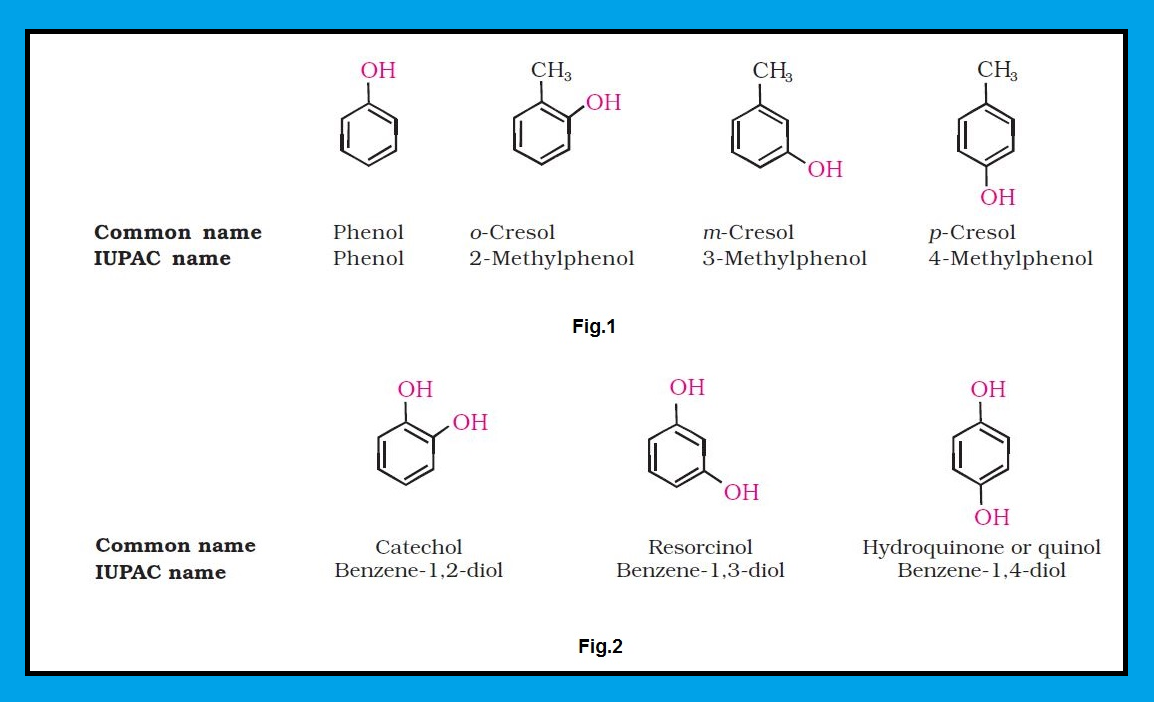
`color{green}("Common Name ")` : The common name of an alcohol is derived from the common name of the alkyl group and adding the word alcohol to it.
● For example, `color{red}(CH_3OH)` is methyl alcohol.
`color{green}("IUPAC Name ")` : According to IUPAC system, the name of an alcohol is derived from the name of the alkane from which the alcohol is derived, by substituting `‘e’` of alkane with the suffix `‘color{red}(ol)’`.
● The position of substituents are indicated by numerals.
● For this, the longest carbon chain (parent chain) is numbered starting at the end nearest to the hydroxyl group.
● The positions of the `color{red}(–OH)` group and other substituents are indicated by using the numbers of carbon atoms to which these are attached.
● For naming polyhydric alcohols, the ‘e’ of alkane is retained and the ending `‘color{red}(ol)’` is added.
● The number of `color{red}(–OH)` groups is indicated by adding the multiplicative prefix, di, tri, etc., before `‘color{red}(ol)’`.
● The positions of `color{red}(–OH)` groups are indicated by appropriate locants e.g., `color{red}(HO–CH_2–CH_2–OH)` is named as ethane–1, 2-diol.
● Table 11.1 gives common and IUPAC names of a few alcohols as examples.
● Cyclic alcohols are named using the prefix cyclo and considering the `color{red}(—OH)` group attached to `color{red}(C–1)`. See image.
● For example, `color{red}(CH_3OH)` is methyl alcohol.
`color{green}("IUPAC Name ")` : According to IUPAC system, the name of an alcohol is derived from the name of the alkane from which the alcohol is derived, by substituting `‘e’` of alkane with the suffix `‘color{red}(ol)’`.
● The position of substituents are indicated by numerals.
● For this, the longest carbon chain (parent chain) is numbered starting at the end nearest to the hydroxyl group.
● The positions of the `color{red}(–OH)` group and other substituents are indicated by using the numbers of carbon atoms to which these are attached.
● For naming polyhydric alcohols, the ‘e’ of alkane is retained and the ending `‘color{red}(ol)’` is added.
● The number of `color{red}(–OH)` groups is indicated by adding the multiplicative prefix, di, tri, etc., before `‘color{red}(ol)’`.
● The positions of `color{red}(–OH)` groups are indicated by appropriate locants e.g., `color{red}(HO–CH_2–CH_2–OH)` is named as ethane–1, 2-diol.
● Table 11.1 gives common and IUPAC names of a few alcohols as examples.
● Cyclic alcohols are named using the prefix cyclo and considering the `color{red}(—OH)` group attached to `color{red}(C–1)`. See image.